A smarter, faster, and
more flexible way to
control batch processes
for modern manufacturers.
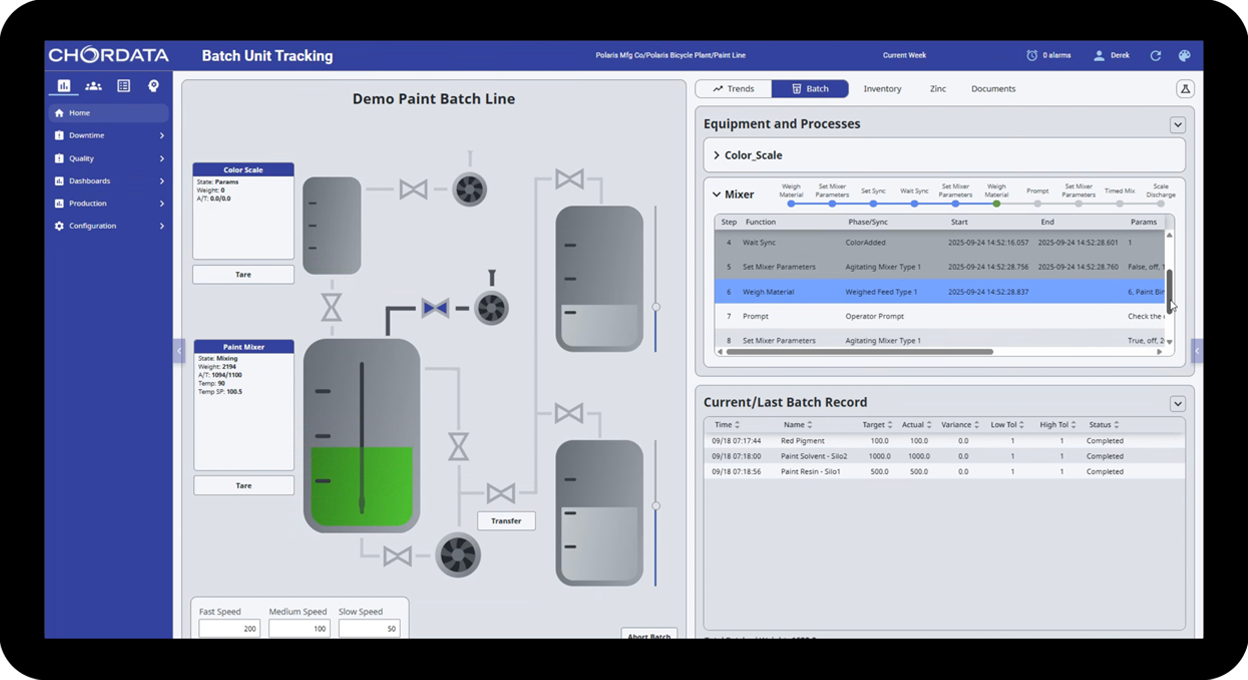
Chordata Batch is purpose-built for manufacturers in highly regulated industries — like pharmaceuticals, food & beverage, and chemicals — who need precise control, complete traceability, and compliance without compromise. Chordata Batch offers amazing flexibility – whether you’re completely rebuilding a legacy system or simply modernizing it by replacing only the batch component.